Publications
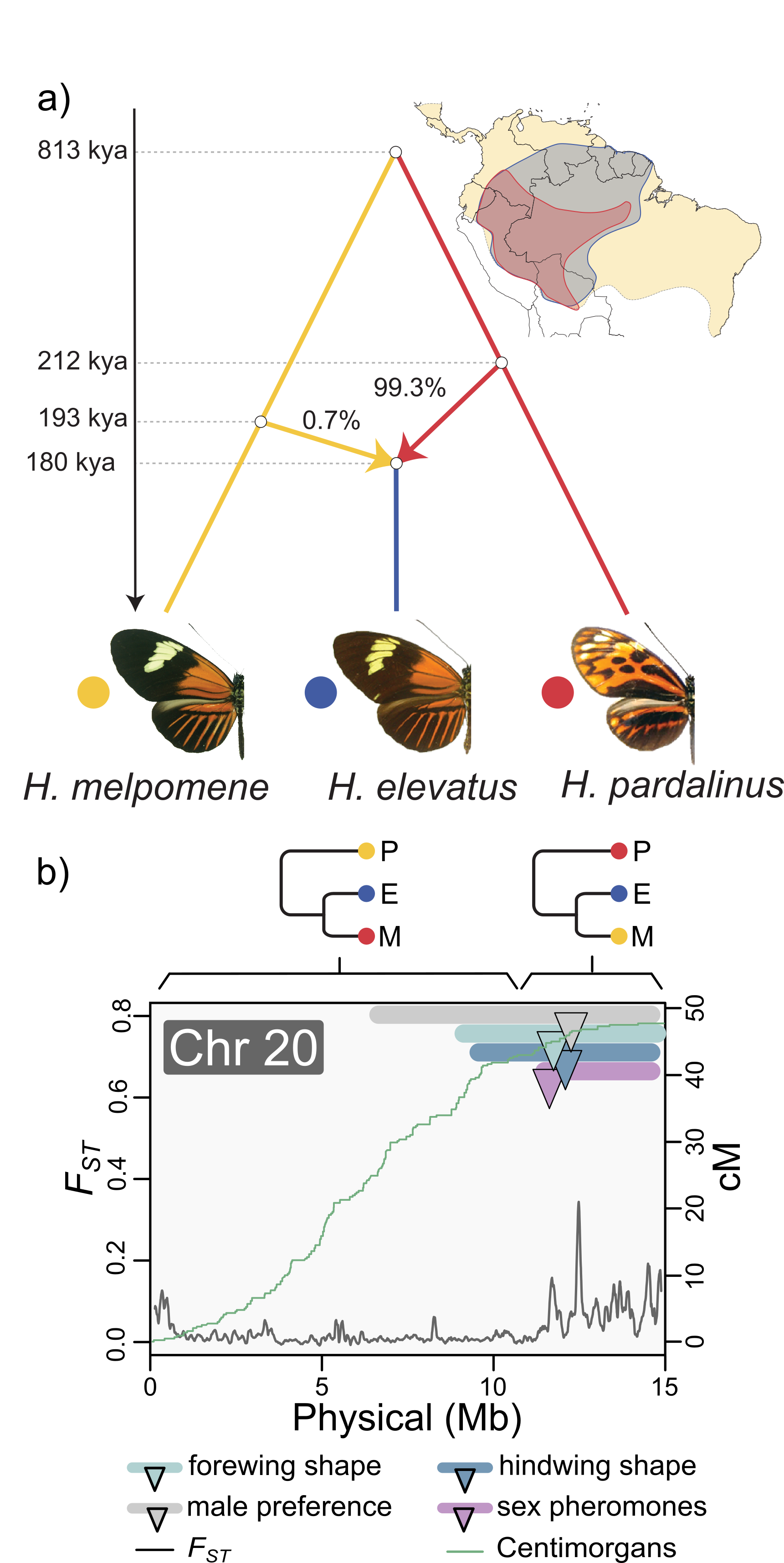
Rosser N, Seixas F + 23 authors + Mallet, J & Dasmahapatra KK. Hybrid speciation driven by multilocus introgression of ecological traits (2024). Nature In press.
Page E, Queste L, Rosser N, Mallet J, Srygley RB, McMillan OW & Dasmahapatra KK. Pervasive mimicry in flight behaviour among aposematic butterflies (2024). PNAS 121(11).
Xiong T, Tarikere S, Rosser N, Li X, Yago M & Mallet J. A polygenic explanation for Haldane’s rule in butterflies (2023). PNAS 120(44).
Costa M, Viloria AL, Neild AFE, Rosser N, Attal S, Benmesbah M & Lamas G. Lepidoptera from the Pantepui. Part XIV. A new subspecies of Heliconius elevatus Nöldner, 1901 (2023). Antenor 10 (2): 56-72.
Sierra-Botero L, Calonje M, Robbins RK, Rosser N, Pierce NE, López-Gallego C & Valencia-Montoya WA. (2023). Cycad phylogeny predicts host plant use of Eumaeus butterflie. Ecology and Evolution 13:e9978.
Xiong T, Tarikere S, Rosser N, Li X, Yago M & Mallet J (2022). Diverse genetic architectures on the Z chromosome underlie the two rules of speciation in swallowtail butterfly hybrids. bioRxiv doi: https://doi.org/10.1101/2022.10.28.514284.
Naka LN, Werneck F, Rosser N, Pil MW & Boubli JP (2022). The Role of Rivers in the Origins, Evolution, Adaptation, and Distribution of Biodiversity. Frontiers in Ecology and Evolution 10:1035859.
Rosser N, Seixas F & Mallet J (2022). Sympatric speciation by allochrony? Molecular Ecology 31:3975–3978.
Rosser N, Edelman N, Queste LM, Nelson M, Seixas F, Dasmahapatra KK & Mallet J (2022). Complex basis of hybrid female sterility and Haldane’s rule in Heliconius: butterflies: Z-linkage and epistasis. Molecular Ecology 31(3):959-977.
Rosser N, Shirai LT, Dasmahapatra KK, Mallet J & Freitas AV (2021). The Amazon river is a suture zone for a polyphyletic group of co-mimetic heliconiine butterflies. Ecography 44(2):177-187.
Rosser N, Queste LM, Cama B, Edelman NB, Mann F, Mori Pezo R, Morris J, Segami C, Velado P, Schulz S, Mallet J & Dasmahapatra KK (2019). Geographic contrasts between pre- and postzygotic barriers are consistent with reinforcement in Heliconius butterflies. Evolution 73(9):1821-1838.
Freitas AVL, Ramos RR, Silva-Brandão KL, Coutoné MN, Magaldi LM, Rosser N & Brown KS (2019). A new subspecies of Heliconius hermathena (Nymphalidae: Heliconiinae) from Southern Amazonia. Neotropical Entomology 48(3):467-475.
Rosser N, Freitas AVL, Huertas B, Joron M, Lamas G, Mérot C, Simpson F, Willmott KR, Mallet J and Dasmahapatra KK (2019). Cryptic speciation associated with geographic and ecological divergence in two Amazonian Heliconius butterflies. Zoological Journal of the Linnean Society 186(1):233-249.
Bernal, XE et al. Empowering Latina scientists (2019). Science 363:825-826.
Rosser N & Mori Pezo R (2017). Colour pattern divergence in Napeocles jucunda Hübner, 1808 is associated with shifts in behaviour, host plant and habitat. Tropical Lepidoptera Research 27(2):106-110.
Schulz S, Vanjari S, Rosser N, Mann S, Dasmahapatra K, Linares M, Pardo-Diaz C, Salazar C, Jiggins C & Mann F (2017). The scent chemistry of Heliconius wing androconia. Journal of Chemical Ecology 43(9):843-857.
Rosser N (2017). Shortcuts in biodiversity research: What determines the performance of higher taxa as surrogates for species? Ecology and Evolution 7(8):2595-2603.
Arias M, Meichanetzoglou A, Elias M, Rosser N, de-Silva DL, Nay B & Llaurens V (2016) Variation in cyanogenic compounds concentration within a Heliconius butterfly community: does mimicry explain everything? BMC Evolutionary Biology 16:272.
Arias M, le Poul Y, Chouteau M, Boisseau R, Rosser N, Théry M, & Llaurens V. (2016). Crossing fitness valleys: empirical estimation of a fitness landscape associated with polymorphic mimicry. Proceedings of the Royal Society B 283:20160391.
Merrill R et al. (2015). The diversification of Heliconius butterflies: What have we learned in 150 years? Journal of Evolutionary Biology 28(8):1417-38.
Rosser N, Kozak KM, Phillimore AB & Mallet J (2015). Extensive range overlap between Heliconius sister species: evidence for sympatric speciation in butterflies? BMC Evolutionary Biology 15:125.
Rosser N, Dasmahapatra K & Mallet, J (2014). Stable Heliconius butterfly hybrid zones are correlated with a local rainfall peak at the edge of the Amazon basin. Evolution 68(12):3470-84.
Green JP, Leadbeater L, Carruthers JP, Rosser N, Lucas ER & Field J (2013). Clypeal patterning in the paper wasp Polistes dominulus: no evidence of adaptive value in the wild. Behavioural Ecology 24(3):623-633.
Heliconius Genome Consortium (2012). A butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487:94-98.
Rosser N, Phillimore AB, Huertas B, Willmott KR, & Mallet J (2012). Testing historical explanations for gradients in species richness in heliconiine butterflies of tropical America. Biological Journal of the Linnean Society 105:479-497.
Rosser N & Eggleton P (2012). Can higher taxa be used as a surrogate for species-level data in biodiversity surveys of litter/soil insects? Journal of Insect Conservation 16:87-92.
Leadbeater E, Carruthers JM, Green JP, Rosser N & Field J (2011). Nest inheritance is the missing source of direct fitness in a primitively eusocial insect. Science 333:874-876.